Trajectory SP960
Force field:
martini_v2.2P
Simulation length (ns): 5000
Electric field (kJ mol-1 nm-1 e-1): 0
Temperature (K): 300 (v-rescale)
Pressure (bar): 1 (Parrinello-rahman semiisotropic)
Number of particles: 39857
Time step (fs) : 25
Software: GROMACS 2021.5
Simulation length (ns): 5000
Electric field (kJ mol-1 nm-1 e-1): 0
Temperature (K): 300 (v-rescale)
Pressure (bar): 1 (Parrinello-rahman semiisotropic)
Number of particles: 39857
Time step (fs) : 25
Software: GROMACS 2021.5
Supercomputer:
Finisterrae III CESGA
Peptides: P269 AP01016
Lipids: POPE, POPG
Heteromolecules:
Ions: NA
Water model: PW
Peptides: P269 AP01016
Lipids: POPE, POPG
Heteromolecules:
Ions: NA
Water model: PW
Sequence :
FFGKMKEYFKKFGASFKRRFANLKKRL
Total charge (e): +9
Number of residues: 27
By amino acid: Basic: 10 Acidic: 1 Hydrophobic: 13 Polar: 3 Electrostatic Dipolar Moment (e nm): 2.29
Longitudinal (e nm): 1.87 Transversal (e nm): 1.32 Hydrophobic Dipolar Moment (nm): 7.15
Longitudinal (nm): 6.7 Transversal (nm): 2.51 Secondary structure: Helix
Activity:
Download Files
ITP file. JSON file. PDB file.
Click on any component to highlight it from the plot.
Upper leaflet
Lower leaflet
Lipids
Membrane model for: POPG:POPE (1:3) (Gram-negative bacteria)
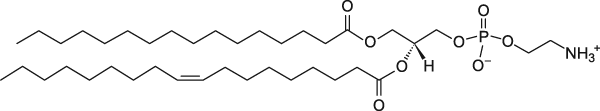
POPE
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
Total charge (e): 0
See POPE lipid
Download ITP File. Download PDB File.
POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol
Total charge (e): -1
See POPG lipid
Download ITP File. Download PDB File.
Last snapshot
Total contacts per residue
Molecular Dynamics based descriptors
Average and standard deviation,
calculated using the autocorrelation function (for time
series)
or the width of the distribution, for the last microsecond
of
the trajectory
Area per lipid
Membrane (nm2): 0.63735400 ± 0.00103814
Upper leaflet (nm2): 0.63735400 ± 0.00103814
Lower leaflet (nm2): 0.63735400 ± 0.00103814
Average Z coordinate
Peptide (nm): 7.979180 ± 0.035975
First Residue (nm): 7.8816900 ± 0.0473608
Last Residue (nm): 7.8914800 ± 0.0478043
Membrane (nm): 6.03610000 ± 0.00951534
Upper leaflet Head Group (nm): 8.0296400 ± 0.0116117
Lower leaflet Head Group (nm): 4.04552000 ± 0.00757298
Bilayer Thickness (nm): 3.984120 ± 0.013863
Peptide insertion (nm): -0.0504516 ± 0.0378026
Contacts
Peptide - Water: 0.0 ± 0.0
Peptide - Head groups: 17.592500 ± 0.304292
Peptide - Tail groups: 15.737500 ± 0.273043
Tilt (°): 89.62530 ± 0.82444
Membrane (nm2): 0.63735400 ± 0.00103814
Upper leaflet (nm2): 0.63735400 ± 0.00103814
Lower leaflet (nm2): 0.63735400 ± 0.00103814
Average Z coordinate
Peptide (nm): 7.979180 ± 0.035975
First Residue (nm): 7.8816900 ± 0.0473608
Last Residue (nm): 7.8914800 ± 0.0478043
Membrane (nm): 6.03610000 ± 0.00951534
Upper leaflet Head Group (nm): 8.0296400 ± 0.0116117
Lower leaflet Head Group (nm): 4.04552000 ± 0.00757298
Bilayer Thickness (nm): 3.984120 ± 0.013863
Peptide insertion (nm): -0.0504516 ± 0.0378026
Contacts
Peptide - Water: 0.0 ± 0.0
Peptide - Head groups: 17.592500 ± 0.304292
Peptide - Tail groups: 15.737500 ± 0.273043
Tilt (°): 89.62530 ± 0.82444
PepDF:
5(ns): CVS
Displacement (nm): 0.5293900 ± 0.0210607
Precession(°): -0.927549 ± 0.831455
50(ns) CVS
Displacement (nm): 1.5490800 ± 0.0744725
Precession(°): -8.42143 ± 2.36450
100(ns) CVS
Displacement(nm): 2.212740 ± 0.104041
Precession(°): -17.66600 ± 3.00007
200(ns) CVS
Displacement(nm): 3.018900 ± 0.186081
Precession(°): -40.48050 ± 4.84594
Download JSON File.
5(ns): CVS
Displacement (nm): 0.5293900 ± 0.0210607
Precession(°): -0.927549 ± 0.831455
50(ns) CVS
Displacement (nm): 1.5490800 ± 0.0744725
Precession(°): -8.42143 ± 2.36450
100(ns) CVS
Displacement(nm): 2.212740 ± 0.104041
Precession(°): -17.66600 ± 3.00007
200(ns) CVS
Displacement(nm): 3.018900 ± 0.186081
Precession(°): -40.48050 ± 4.84594
Download JSON File.
Peptide Analyses
Peptide Displacement Fingerprint
(PepDF)
Lateral displacement vs
Rotational
Displacement along the trajectory, for different time
windows .
Density maps:
2D-density maps of lipids around the
peptide
along XY and YZ axis, calculated for each lipid type along the
last
microsecond.
Lipid-Peptide Analyses:
z-Position
Z-coordinate, averaged for
differetn
parts of the the system: peptide, membrane, first and
last
backbone (BB) residues and upper of lower leaflet
lipids’
headgroups (HGs).
Minimum distance
Minimum distance (nm) between the
peptide backbone and the lipids (headgroups and
tailgroups).
Number of contacts
Number of contacts between the
peptide backbone and the water or the lipids separated
by
lipid headgroups (HG) or lipid tails, using a cut-off of
0.6
nm.
Lateral density
Lateral density for the different
components of the system: headgroups, tail groups,
peptide
and water.